Novavax: Everything You Need to Know About the Fourth COVID-19 Vaccine
Novavax has created a fourth COVID-19 vaccine using moth cells and tree bark. Instead of an mRNA vaccine such as Pfizer and Moderna, or a viral vector vaccine like Johnson & Johnson, Novavax is a subunit protein vaccine. But, what does it mean? According to Gavi the Vaccine Alliance, subunit vaccines have portions of ‘protein and/or polysaccharide from the pathogen’. By restricting the immune system’s access to the pathogen in this way, the risk of side effects is minimized. These vaccines are also relatively cheap and easy to produce, and more stable than those containing whole viruses or bacteria.
Differences Between Novavax And the Other COVID-19 Vaccines
According to Connecticut State, both mRNA and viral vector vaccines contain instructions that teach our cells how to create “spike proteins”, which is the protein found on the surface of the virus that causes COVID-19. Once your cells produces COVID-19 spike proteins, your immune system recognizes that those proteins don’t belong in your body and creates antibodies to stop the virus from spreading and causing damage when you are exposed to it. Neither vaccine contains the virus that causes COVID-19.
The instructions in the mRNA vaccines are messenger RNA (mRNA), the genetic material that tells your cells how to make proteins. The mRNA is surrounded by tiny lipids (fatty molecules) which help mRNA enter directly into your cells. Once your cells create the spike proteins, your body breaks down the mRNA. In viral vector vaccines, spike protein DNA is placed inside a modified version of a different virus that doesn’t cause illness. This non-harmful virus delivers the DNA instructions to your cells – this virus is called the vector.
Infectious diseases expert Diana Florescu, MD, led the phase 3 clinical trial of the Novavax vaccine at the University of Nebraska Medical Center (UNMC). “Diversity in vaccine production helps increase the number of patients vaccinated,” says Dr. Florescu. “Some might not accept mRNA vaccines, while others may be allergic to certain ingredients. For example, some are allergic to polyethylene glycol (PEG), an ingredient in the mRNA (Pfizer and Moderna) vaccines. So, when are we going to start seeing the Novavax among the population?
Novavax Begins Shipping COVID Vaccine to Europe
Novavax Inc. has shipped the first doses of its Covid-19 vaccine to Europe, marking the drug company’s entry into a potentially large new market. According to Novavax President & CEO Stanley Erck, this is a big milestone for the company, and argues that the vaccine could be approved in Canada and the United States. NVX-CoV2373 is currently being evaluated in two pivotal Phase 3 trials: a trial in the U.K that completed enrollment in November 2021, and the PREVENT-19 trial in the U.S. and Mexico that began in December 2021. It is also being tested in two ongoing Phase 2 studies that began in August: a Phase 2b trial in South Africa, and a Phase 1/2 continuation in the U.S. and Australia.
Novavax produced the NVX-CoV2373, a vaccine candidate that is designed to provide protection against COVID-19 on the long run, especially against potential new variants. Engineered from the genetic sequence of COVID-19, Novavax used their recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein. In combination with the proprietary Matrix-M™ adjuvant, the vaccine has proven in preclinical studies that “it binds efficiently with human receptors targeted by the virus, a critical aspect for effective vaccine protection”. According to Novavax representatives, it will only be a matter of time to see this new coronavirus vaccine worldwide. Nonetheless, in the meantime, how do we deal with the Omicron variant?
Pfizer’s Omicron Vaccine Will be Ready in March
Pfizer CEO Albert Bourla on Monday said a vaccine that targets the omicron variant of Covid will be ready in March, and the company’s already begun manufacturing the doses. “This vaccine will be ready in March,” Bourla told CNBC’s “Squawk Box.” “We [are] already starting manufacturing some of these quantities at risk.” Bourla said the vaccine will also target the other variants that are circulating. He said it is still not clear whether or not an omicron vaccine is needed or how it would be used, but Pfizer will have some doses ready since some countries want it ready as soon as possible. “The hope is that we will achieve something that will have way, way better protection particularly against infections, because the protection against the hospitalizations and the severe disease — it is reasonable right now, with the current vaccines as long as you are having let’s say the third dose,” Bourla said.
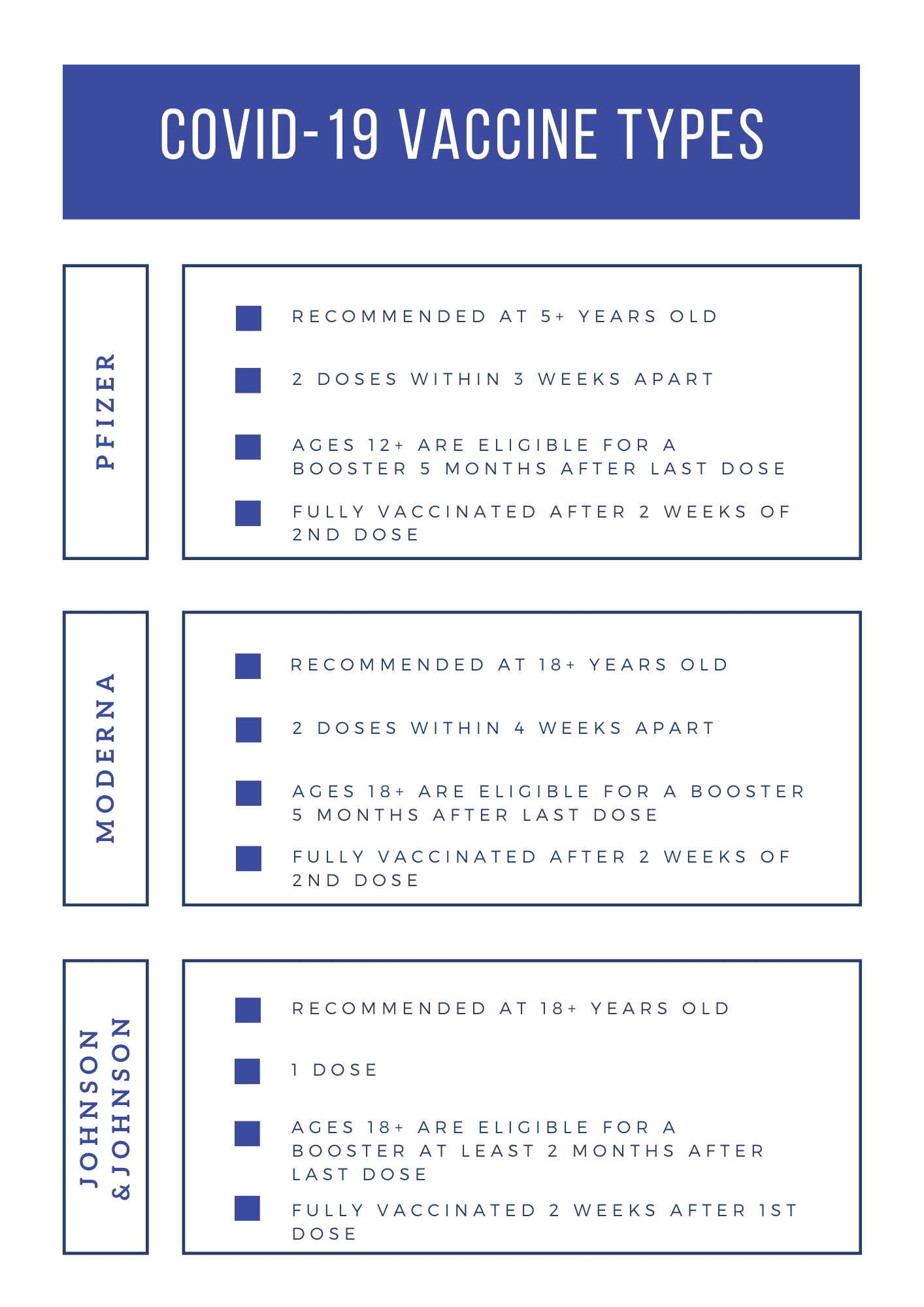
According to the Centers for Disease Control and Prevention, Three COVID-19 vaccines are authorized or approved for use in the United States to prevent COVID-19. Pfizer-BioNTech or Moderna (COVID-19 mRNA vaccines) are preferred. Moreover, you may get Johnson & Johnson’s Janssen COVID-19 vaccine in some situations. However, one of the main problems that COVID vaccines have faced during 2021 was its distribution among low income countries. The COVID-19 pandemic affects every single person, regardless of their age, gender, ethnicity, income, or any other demographic. Furthermore, there have been several attempts of negotiations and treaties in order to distribute different vaccines around the world for those countries in need. So, at this point you might be wondering, is there a way to have an affordable number of vaccines to administrate worldwide to every single country in the Earth with no restrictions?
Global Game Changer Texas COVID-19 Vaccine
A vaccine authorized in December for use in India may help solve one of the most vexing problems in global public health: How to supply lower-income countries with a COVID-19 vaccine that is safe, effective and affordable. The vaccine is called CORBEVAX. It uses old but proven vaccine technology and can be manufactured far more easily than most, if not all, of the COVID-19 vaccines in use today. “It’s going to enable countries around the world, particularly low-income countries, to be able to produce these vaccines and distribute them in a way that’s going to be affordable, effective and safe.” says Dr. Keith Martin, executive director of the Consortium of Universities for Global Health in Washington, D.C.
The story of CORBEVAX begins some two decades ago. Peter Hotez and Maria Elena Bottazzi were medical researchers at George Washington University in Washington, D.C., where they worked on vaccines and treatments for what are called neglected tropical diseases, such as schistosomiasis and hookworm. When a strain of coronavirus known as SARS broke out in 2003, they decided to tackle that disease. After moving to Houston to affiliate with Baylor College of Medicine and the Texas Children’s Center for Vaccine Development, they created a vaccine candidate using protein subunit technology. This involves using proteins from a virus or bacterium that can induce an immune response but not cause disease.
Regardless of this potential new “game changer” vaccines, it seems to be that the potential future variants will continue to appear. That means, that every now and then, people might have to reinforce their immune system with another COVID-19 vaccine. Is there an end to that back-and-forth scenario?
The Million Dollar Question: Will ‘Forever Boosting’ Beat the Coronavirus?
A year ago, just two doses of a Covid-19 vaccine — or even one, in the case of Johnson & Johnson’s formulation — were thought to offer sufficient protection against the coronavirus. Now, faced with the extraordinarily contagious Omicron variant, Israel has begun offering fourth doses to some high-risk groups. In January 2022, the Centers for Disease Control and Prevention expanded eligibility for boosters to adolescents and backed away from describing anyone as “fully vaccinated” because two shots no longer seem adequate.
Instead, one’s vaccination status will now be “up to date” — or not. It’s no surprise that many Americans are wondering: Where does this end? Are we to roll up our sleeves for booster shots every few months? Humbled repeatedly by a virus that has defied expectations, scientists are reluctant to predict the future. But in interviews this week, nearly a dozen said that whatever happens, trying to boost the entire population every few months is not realistic. Nor does it make much scientific sense.
Are There Any Side Effects From the Novavax Vaccine?
There is no official record of a side effect coming from the Novavax vaccines as for January 2022. However, here at Malloy Law Offices, LLC we have seen SIRVA cases related to COVID-19 vaccination process. SIRVA stands for “Shoulder Injury Related to Vaccine Administration”, and it happens when a vaccine is injected too high or too deep in the shoulder. This negligent act might lead to intense and ongoing pain in the shoulder and arm. Moreover, it could cause other shoulder injuries, such as a rotator cuff tear or tendonitis. That is why it is always better to talk to a Vaccine Injury Lawyer. If you have any questions related to COVID-19 vaccines, feel free to call our office: (202) 933-1918.